High Purity New England is proud to announce that we are now a Getinge company. Learn More.
We are now members of Rx-360. Learn more about the advantages that provides for our customers. Learn More.
Featured Product Categories
Find it fast—get it fast.
Featured Products & Services
Solutions for bioprocessing challenges
Media & Buffer
Storage Solutions
Streamline your media and buffer storage with products, including our Harvest Carts, Custom Tubing Trolley, and more.

Meet OptiMaxx:
Our New TFF System
Designed specifically to automate your applications, our tangential flow filtration systems are perfect for your unique processes.


Sterile Individually
Packaged Components
Save time and increase
productivity with pre-sterilized,
individually double-bagged
grab-it-and-go components.
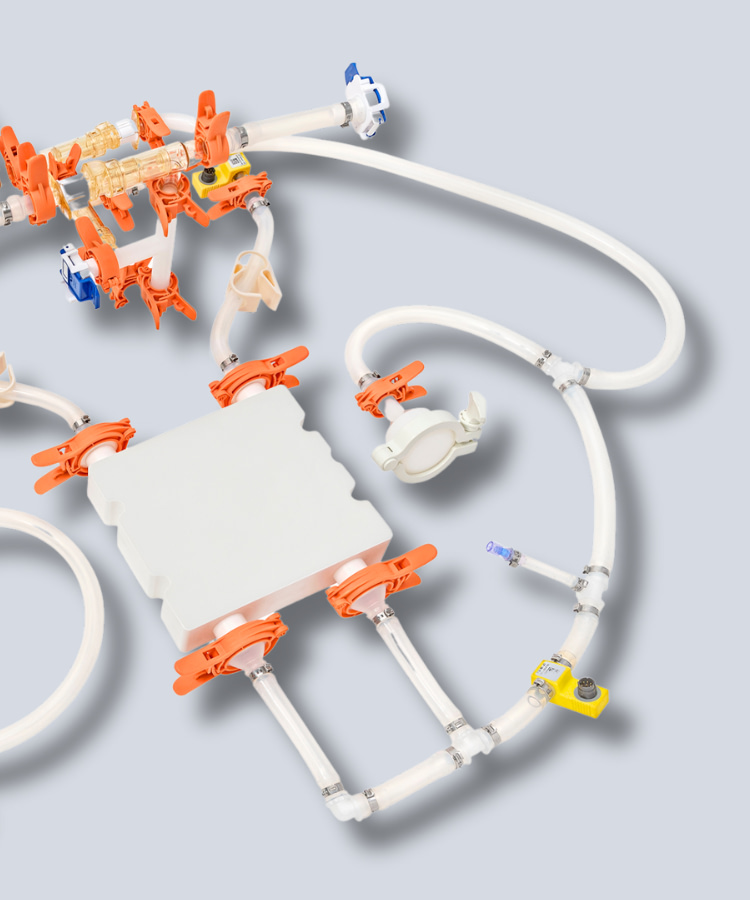
HPConnexx™
Single-Use Assemblies
Fast lead times, no minimum order quantity, and the ability to truly customize your assembly using any commercial available part.
Pump + Sensor =
Results
Simplify your setup and increase productivity when you integrate flow and pressure sensors with a Quattroflow pump.

Flow, Pressure, UV & Other Sensors
Explore our wide range of both multi-use and single-use sensors, perfect for incorporating into new and existing bioprocesses

Products By Application Area
Upstream Bioprocessing
From cell culture development to harvest and collection, HPNE has a range of tailored solutions to improve your process efficiency and maximize productivity.
Downstream Bioprocessing
Optimize the downstream recovery and purification of your products with customizable solutions for every scale.
Lab and Drug Discovery
Find bioprocessing solutions that scale as you scale, from bench-top discovery and initial proof-of-concept to pilot scale and commercialization.
Fill and Finish
Ensure the integrity of delicate biologics at this last stage before shipping with solutions that meet demanding sterility and quality specifications.
Meet HPNE
Solving Bioprocessing Challenges Through
Innovative Engineering Since 2002
Since our founding in 2002, the biopharmaceutical industry has experienced incredible growth and technological change, and HPNE has remained at the forefront of innovation. We’ve helped countless companies streamline and innovate their bioprocesses as well as develop new production lines and scale up.
No matter how much the landscape changes, one thing has remained constant over the years, our commitment to quality. From our engineering solutions, to the products we distribute and the products we manufacture, in every customer interaction, our goal is to deliver excellence and quality.